VOID LABELS & TAPES
For all our security seal, security tape and security labels, the VOID-effect can be fully customized. Thereby it represents a security feature on its own and increases the level of security compared to generic, freely available products that can be used by anyone. VOID security seal, tape & labels can be applied with your existing labelling equipment and typically perform well on all kinds of substrates. Due to these benefits, VOID anti tamper seals provide superior performance than other solutions.

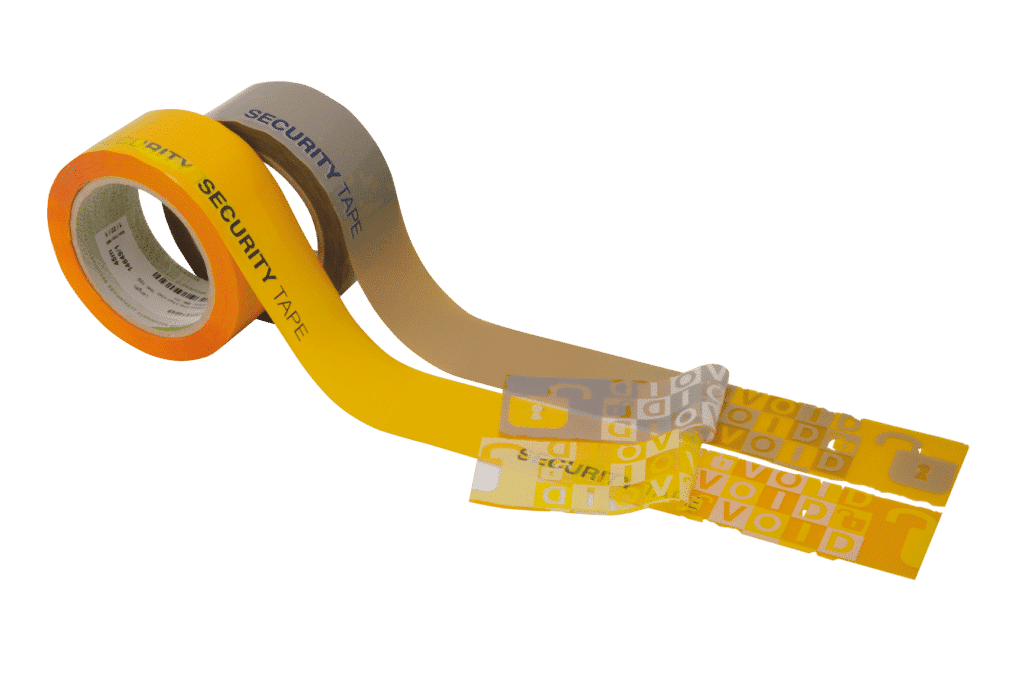
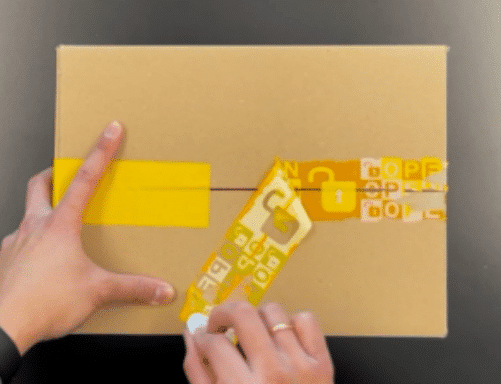
SECURITY FUNCTIONS
- Simple and intuitive unpackaging
- Custom-designed Void effects
- Compliant with FMD safety requirements
- Make your packaging sustainable
- Available in all sizes and shapes
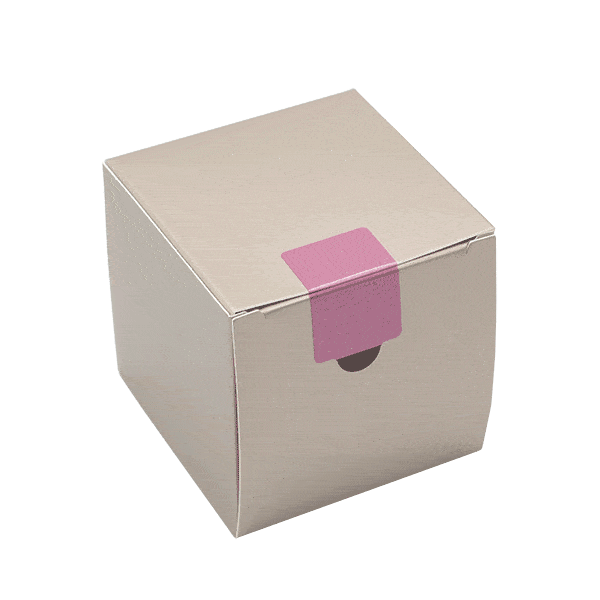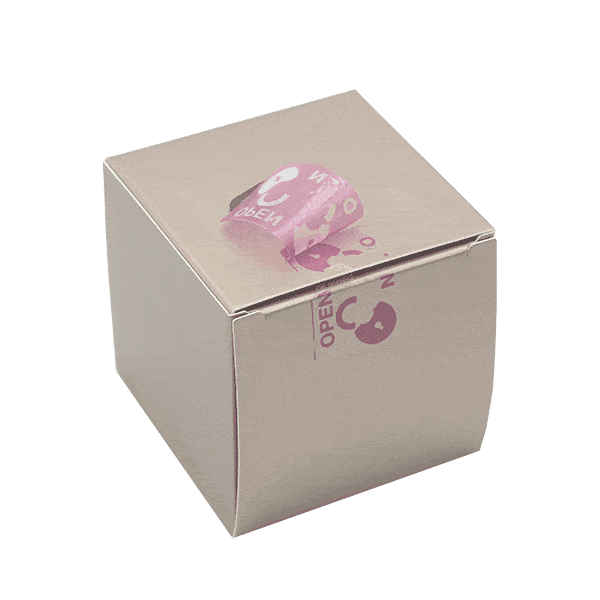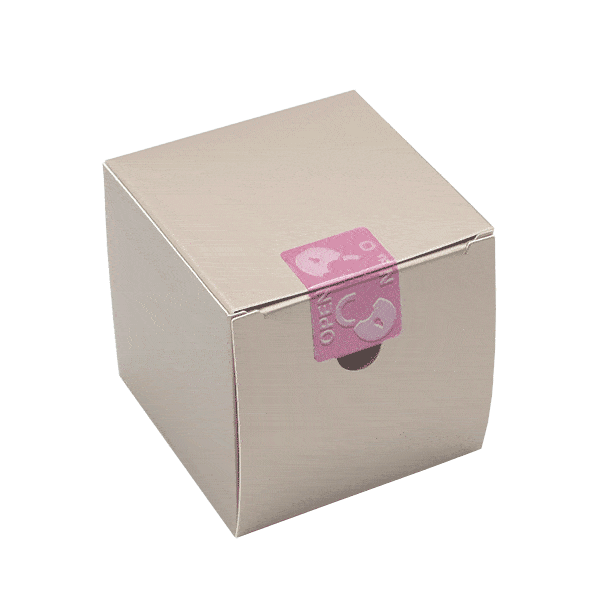
Our paper tamper evident seals, labels and tapes are easy to understand. And the unexpected opening effect will surprise your consumers. To make the unboxing experience of your sustainable packaging perfect, we provide opening tabs for easy peel-off.
Arrow symbols, texts and colored areas make it obvious where the recyclable labels should be peeled off. Playful elements on the sustainable product packaging encourage the consumer to scan the QR-code on the security label.

We create individual VOID effects into our security labels that fit your brand and design. Whether you want to integrate your logo, symbols, or text into the opening effect – with Securikett there are no limits. Our triple-void technology offers surprising effects for our sustainable packaging. Even on dark and bright areas of packaging at the same time.

We work in compliance with the strictest quality guidelines, such as the Falsified Medicines Directive (FMD). This requires the serialization of each individual product, as well as a unique feature for tamper evidence. Our FMD-compliant security seals are also available in paper to complete our sustainable packaging.

The need for eco-friendly solutions for sustainability and environment protection brings the Securikett product portfolio to a new level. Innovative security labels are created under consideration of the 3-R-strategy (reduce, reuse, and recycle). It helps companies with the development of eco-sustainable and recyclable label respectively packaging solutions. With our IT’S PAPER product series, we created a perfect substitution for plastics.
Our paper security labels with the PaperVOID effect allows you to fulfil the requirements of a sustainable and recyclable economy. But also, you can fulfil the requirements regarding security and traceability. The use of resource-efficient materials and innovative adhesives is of great importance to us.
We take the whole life cycle into consideration, including the recycling process of sustainable packaging. As a result, we designed a secure and recyclable label solution for paper-based packaging.

From subtle to conspicuous – we provide neutral, single-color products as well as sparkling or fantasy designs to create your customized security seal. We guarantee that your consumers will be amazed. Our paper security labels and seals are available in different sizes, shapes and colors. All of our products are customized to our customers’ corporate design. This includes not only the external design but also the opening effect of the security label, which only becomes visible when the label is peeled off. There are no limits to your ideas.

HOW TO USE
With our translucent or transparent technologies, any information remains readable and intact, even when fully covered by the security seal.
Moreover, you can also seal your packaging in an environmentally friendly way: our paper-based VOID security tape for packages supports the recyclability of your paper-based packaging.

BENEFITS
EASY AND CLEAR INDICATION OF FIRST OPENING
FMD-COMPLIANT FOR PHARMACEUTICALS
NO CHANGES IN PACKAGING DESIGN
FULLY CUSTOMIZABLE
TRANSFER AND SHIPPING
As an intrinsic security feature, this significantly increases the level of security. Intensive internal stress-level testing (e.g. drop test) has confirmed superior performance of our security seal. In addition, they also work reliably under deep-freeze conditions. Integration in your existing processes has never been so easy: Securikett VOID security tapes work both with customary dispensers and on automated systems (larger machine reels are possible)



GET YOUR SAMPLE SECURITY SEAL
WHAT MAKES OUR TAMPER-EVIDENT SECURITY LABELS SO SPECIAL?
TAMPER-EVIDENCE
Moreover, for all our tamper proof labels, tape and seal, the design of the VOID-effect can be fully customized and thus providing a sophisticated security feature itself. Technologies like triple VOID or high-contrast VOID add stunning effects for this purpose.
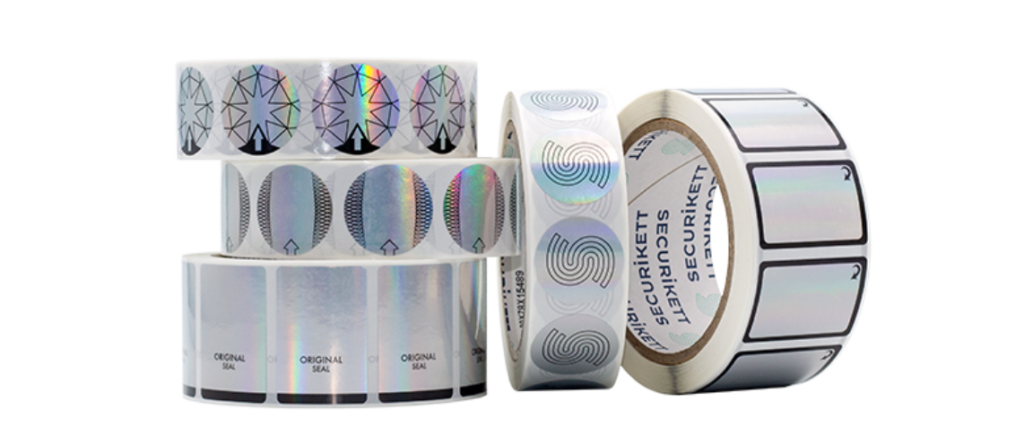
HOLOGRAMS & INKS
NFC AND RFID
UNIQUE IDENTIFIERS
EXAMPLES FOR TAMPER EVIDENT LABELS,
SEALS AND TAMPER TAPE
BEST PRACTICE
TAMPER VERIFICATION
SUSTAINABLE PACKAGING
TAMPER-EVIDENCE FOR ALL PRODUCTS
PRODUCT PROTECTION